Global ADC Drug Revenue TOP10 in 2022
New Media Department Yu Jie, Duan Yuqing March 8, 2023
Up to now, the number of global ADC drugs that has been approved is 15. It is worth mentioning that Elahere, jointly developed by Immunogen and East China Pharmaceutical, was approved for listing on November 14, 2022. It is the first ADC drug approved by FDA for platinum resistant ovarian cancer. Among them, there are 6 products approved in China, namely Roche's Enmei Monzumab, Weibuqin monoclonal of Seagen/Takeda Pharmaceutical, Pfizer's Ogilfuzumab, Vidici monocidal resistance of Rongchang Bio, Immunomedics and Detuszumab jointly developed by Astrikon/first and third co -developed on February 24, 2023.
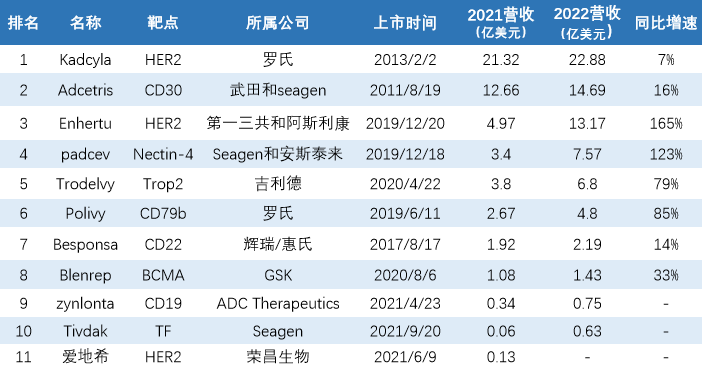
In 2021, we counted 11 ADC drugs with a total sales of $ 5.25 billion. In 2022, 10 drug sales data were announced. The total revenue was about 7.491 billion US dollars, an increase of about 42.69%year -on -year.
