
Valsartan Dispersible Tablets
DescriptionFull Analysis capability
Thin Film Coating Lab
Valsartan Dispersible Tablets
Indication
Essential hypertension.
Clinical pharmacology
Valsartan is an angiotensin II (AngII) receptor antagonist that selectively blocks the binding of AngII to the AT1 receptor (which specifically antagonizes the AT1 receptor by about 20,000 times greater than AT2) and thereby inhibits blood vessels. Contraction and release of aldosterone produce a hypotensive effect. This product does not act on angiotensin-converting enzyme (ACE), renin and other receptors, and does not inhibit ion channels associated with blood pressure regulation and sodium balance. This product does not affect the level of bradykinin in the body, thus causing fewer side effects of cough than angiotensin converting enzyme inhibitors.
Dosage
The recommended starting dose of this product is 80mg (2 tablets), once a day orally. Generally, when the 4 weeks is invalid, the dose can be increased to 160 mg (4 tablets) once a day. Foreign clinical application data reported that the maximum dose can reach 320mg (8 tablets) once a day. Severe hypertension and decreased blood pressure after drug increase are still unsatisfactory. Small doses of diuretics (such as thiazides) or other antihypertensive drugs may be added.
Formulation
Dispersible film
specification
40mg.
Instruction manual
Date of approval: May 8, 2007
Date of revision: April 12, 2013 2016-1-5 (adding packaging specifications)
Valsartan dispersible tablet instruction
Please read the instructions carefully and use them under the guidance of a physician.
【Drug Name】
Generic name: valsartan dispersible tablets
Product Name: Pingxin
English name: Valsartan Dispersible Tablets.
Chinese Pinyin: Xieshatan Fensan pian.
【ingredients】valsartan.
Chemical name: N-(1-oxopentyl)-N-{[2'-(1H-tetraazol-5-yl)-[1,1'-diphenyl]-4-yl]methyl}- L-valine
Chemical Structure:
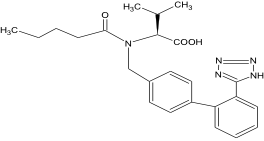
Molecular formula: C24H29N5O3 Molecular weight: 435.52
【Properties】 This product is a white film.
【Indications】 Essential hypertension.
【Specifications】40mg.
【Dosage】
The recommended starting dose of this product is 80mg (2 tablets), once a day orally. Generally, when the 4 weeks is invalid, the dose can be increased to 160 mg (4 tablets) once a day. Foreign clinical application data reported that the maximum dose can reach 320mg (8 tablets) once a day.
Severe hypertension and decreased blood pressure after drug increase are still unsatisfactory. Small doses of diuretics (such as thiazides) or other antihypertensive drugs may be added.
【Adverse reactions】
The reported incidence of adverse reactions in this product was 7.1%, similar to placebo. Common side effects are headache and edema, which are generally mild and transient, and most patients can tolerate it. The incidence of dry cough is significantly reduced compared to ACEI. Other adverse reactions include diarrhea, migraine, occasional increase in transaminases, leukopenia and thrombocytopenia, hyperkalemia, and very few urticaria and angioedema.
【taboo】
1. It is forbidden to allergic to the ingredients of this product.
2. Pregnant and lactating women are banned.
【Precautions】
1. Blood volume deficiency and/or hyponatremia should be corrected before starting treatment.
2. When combined with a potassium-sparing diuretic (such as ampicillin), pay attention to monitoring blood potassium to avoid hyperkalemia.
3, patients with renal insufficiency should pay attention to monitoring changes in urea nitrogen, serum creatinine and serum potassium; mild to moderate renal insufficiency do not need to adjust the dose. People with severe renal insufficiency (creatinine elimination rate <30ml/min) may need to be reduced.
4, patients with liver dysfunction do not need to adjust the dose, but the biliary infarction patients with valsartan clearance rate is reduced, when taking this product, should be particularly careful.
5, healthy people overdose this product may appear hypotension, tachycardia or bradycardia, vomiting, gastric lavage and other supportive therapy. Valsartan cannot be eliminated by hemodialysis.
6. When using in combination with non-steroidal compounds such as diclofenac sodium, check the blood cell counts such as platelets.
【Pregnant women and lactating women medication】disabled.
【Children's medication】 There is no information on the safety of medication for patients under 18 years of age.
【Geriatric Use】No need to adjust the dose.
【medicine interactions】
There is no obvious difference between this product and diuretics (hydrochlorothiazide), cardiac sputum (such as digoxin), ß receptor blockers (such as atenolol), calcium antagonists (such as nifedipine) and Huafalin. interaction.
Patients with insufficient blood volume should prevent hypotension when used in combination with diuretics. Avoid hyperkalemia when combined with a potassium-sparing diuretic (amphetamine).
【Drug overdose】Although there is no experience in overdose of sartan, the main symptom that may occur in drug overdose is obvious hypotension. If it occurs shortly after taking the drug, vomiting may be taken. Otherwise, intravenous saline may be administered as usual. Hemodialysis does not seem to clear Valsartan.
【Pharmacology and Toxicology】
Pharmacological action
Valsartan is an angiotensin II (AngII) receptor antagonist that selectively blocks the binding of AngII to the AT1 receptor (which specifically antagonizes the AT1 receptor by about 20,000 times greater than AT2) and thereby inhibits blood vessels. Contraction and release of aldosterone produce a hypotensive effect. This product does not act on angiotensin-converting enzyme (ACE), renin and other receptors, and does not inhibit ion channels associated with blood pressure regulation and sodium balance. This product does not affect the level of bradykinin in the body, thus causing fewer side effects of cough than angiotensin converting enzyme inhibitors.
Toxicological research
Repeated administration toxicity: Rats were given valsartan daily at a dose of 60, 200, 600 mg/Kg for three consecutive months. The water intake and hematuria of the high-dose group increased slightly, and the male rats were mild. Glomerular hyperplasia and basophils increased, and glomerular input into the small arteries of female rats. No abnormalities were observed after the recovery period. The highest dose of valsartan in rats was 200mg/Kg/day (some animals for 6 months). For 12 consecutive months, the highest dose of oral valsartan in monkeys was 120mg/Kg/day for 12 months. Adverse reactions.
Genotoxicity: The microbial reverse mutation test (Ames test) of this product, the results of Chinese hamster V79 cell gene mutation test, Chinese hamster ovary cell chromosome test and rat cytogenetic test were all negative.
Reproductive toxicity: oral dose of this product in pregnant rats or mice up to 600mg / Kg / day, oral dose of pregnant rabbits up to 10mg / Kg / day, no teratogenic effects. However, when the mother rats were given a dose of 600 mg/kg/day during the organogenesis or late pregnancy and lactation, the weight of the rat fetus decreased, the fetal survival rate decreased, and the fetal development was delayed. Fetal toxicity (absorption of fetuses, reduction in litter size, abortion, and weight loss) in rabbits is associated with toxicity at maternal doses of 5 mg/kg/day and 10 mg/kg/day. The mice were not observed at 2, 200 and 600 mg/Kg/day, and rats and rabbits were 0.1, 6, and 9 times the maximum recommended dose (calculated as 60 kg body weight, dose 320 mg/kg/day). Adverse reactions.
Carcinogenicity: mice and rats were given 160 and 200 mg/kg/day for two consecutive years, 2.6 and 6 times of the maximum recommended dose (calculated as 60 kg/body weight, 320 mg/kg/day). Carcinogenic effect.
【Pharmacokinetics】
According to foreign clinical research data, this product absorbs quickly after oral administration, the absolute bioavailability is 25%, and the absorption rate after taking the food is reduced by 46%, but the clinical effect is not significantly reduced, so it can be taken before and after meals. The plasma peak time is 2 to 3 hours, the elimination half-life is 6 to 7 hours, and the plasma protein binding rate is 94 to 97%. Take the drug once a day, and the blood concentration reaches steady state after three days. Valsartan is metabolized by the prototype drug, mainly (70%) excreted through the biliary tract and the rest excreted through the kidney.
【Storage】Sealed and stored in a dry place (not exceeding 30 ° C).
【Packaging】Aluminum plastic packaging. 14 pieces / box, 24 pieces / box, 28 pieces / box.
【Validity Period】24 months.
【Executive Standards】National Food and Drug Administration National Drug Standard WS1-(X-003)-2009Z
【Approval No.】 National Pharmaceutical Standard H20051350
【manufacturer】
Company Name: Lunan Beite Pharmaceutical Co., Ltd.
Production address: No. 243, Yinqueshan Road, Linyi City, Shandong Province
Postal code: 276006
Phone number: 0539-8336336 (Sales) 0539-8336337 (Quality Pipe Department)
Fax number: 0539-8336029 (Sales) 0539-8336338 (Quality Pipe Department)
Website: www.LUNAN.com.cn
24-hour customer service hotline: 400-0539-310
