Zoledronic Acid for Injection
DescriptionFull Analysis capability
Thin Film Coating Lab
Zoledronic Acid for Injection
Indication
Bone pain caused by osteolytic bone metastasis of malignant tumor
Clinical pharmacology
The pharmacological action of zoledronic acid is mainly to inhibit bone resorption, and its mechanism of action is not fully understood, and may be related to various effects. Zoledronic acid inhibits osteoclast activity, induces osteoclast apoptosis, and blocks osteoclast absorption of mineralized bone and cartilage by binding to bone. Zoledronic acid also inhibits osteoclast activity and bone calcium release caused by multiple stimuli released by the tumor.
Dosage
Intravenous drip. Adults each time 4mg, diluted with 100ml 0.9% sodium chloride injection or 5% glucose injection, intravenous infusion, the infusion time should be no less than 15 minutes. Dosage every 3 to 4 weeks or as directed by your doctor.
Formulation
Powder needle
specification
4mg (according to C5H10N2O7P2)
Instruction manual
Approval date: April 22, 2007
Date of revision: August 29, 2014
Instructions for zoledronic acid for injection
Please read the instructions carefully and use them under the guidance of a physician.
【Drug Name】
Generic name: zoledronic acid for injection
Product Name: In Lida
English name: Zoledronic Acid for Injection
Pinyin: Zhusheyong Zuolailinsuan
【Ingredients】 The main component of this product is zoledronic acid.
Chemical name: [1-hydroxy-2-(1-imidazolyl)ethylidene diphosphate monohydrate.
Chemical Structure:
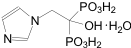
Molecular formula: C5H10N2O7P2·H2O Molecular weight: 290.1
【Properties】 This product is white or off-white loose mass or powder.
【Indications】 Bone pain caused by osteolytic bone metastasis of malignant tumors.
【Specification】 4mg (according to C5H10N2O7P2).
【Usage and dosage】 intravenous drip. Adults each time 4mg, diluted with 100ml 0.9% sodium chloride injection or 5% glucose injection, intravenous infusion, the infusion time should be no less than 15 minutes. Dosage every 3 to 4 weeks or as directed by your doctor.
【Adverse reactions】
The most common adverse reaction of this product is fever, and other adverse reactions mainly include:
Systemic reactions: fatigue, chest pain, leg edema, conjunctivitis;
Digestive system: nausea, vomiting, constipation, diarrhea, abdominal pain, difficulty swallowing, anorexia;
Cardiovascular system: hypotension;
Blood and lymphatic system: anemia, hypokalemia, hypomagnesemia, hypophosphatemia, hypocalcemia, neutropenia, thrombocytopenia, whole blood cell reduction;
Musculoskeletal: bone pain, joint pain, muscle pain;
Kidney: elevated creatinine levels in serum (related to the time of administration);
Nervous system: insomnia, anxiety, excitement, headache, somnolence;
Respiratory system: difficulty breathing, cough, pleural effusion;
Infection: urinary tract infection, upper respiratory tract infection;
Metabolic system: anorexia, weight loss, dehydration;
Other: flu-like symptoms; redness, rash, itching, etc. at the injection site.
The toxic side effects of zoledronic acid are mostly mild and transient, and in most cases will automatically resolve within 24 to 48 hours without special treatment.
【taboo】
1. Disabled patients who are allergic to this product and other bisphosphonates.
2, severe renal insufficiency is not recommended.
3. Pregnant women and lactating women are banned.
【Precautions】
1. When using this product for the first time, the serum levels of calcium, phosphorus, magnesium and serum creatinine should be closely monitored. If the serum levels of calcium, phosphorus and magnesium are too low, the necessary supplementary treatment should be given;
2, patients with malignant hypercalcemia should be adequately hydrated before administration of this product, diuretics and this product can only be used after adequate hydration, this product should be used with the drug with nephrotoxicity should be cautious;
3. When receiving this product, if there is deterioration of renal function, the drug should be discontinued until the renal function returns to the baseline level;
4. This product should be used with caution in asthma patients who are allergic to aspirin.
【Pregnant women and lactating women】
Whether this product will be secreted into the milk is not clear, because this product can be combined with bone for a long time, pregnant women and lactating women disable this product.
【Children's medication】
The safety and effectiveness of this product in children has not been established and is not recommended for use.
【Geriatric Use】
Use with adults. However, elderly patients often have lower renal function, and renal function should be closely monitored during drug administration.
【medicine interactions】
This product should be used with aminoglycosides in combination, because aminoglycosides have a synergistic effect of lowering blood calcium, which may prolong the duration of hypocalcemia; when combined with diuretics, it may increase the risk of hypocalcemia; When combined with thalidomide, it increases the risk of renal dysfunction in patients with multiple myeloma.
【Drug overdose】
Patients receiving high doses of this product may cause levels of calcium, phosphorus and magnesium in the serum to be too low, which can be supplemented by intravenous administration of calcium gluconate, potassium or sodium phosphate and magnesium sulfate. In addition, high doses of this product increase the risk of nephrotoxicity. The single dose of zoledronic acid should not exceed 4 mg.
【Pharmacology and Toxicology】
Pharmacological action
The pharmacological action of zoledronic acid is mainly to inhibit bone resorption, and its mechanism of action is not fully understood, and may be related to various effects. Zoledronic acid inhibits osteoclast activity, induces osteoclast apoptosis, and blocks osteoclast absorption of mineralized bone and cartilage by binding to bone. Zoledronic acid also inhibits osteoclast activity and bone calcium release caused by multiple stimuli released by the tumor.
Toxicological research
Genotoxicity:
The product Ames bacterial back mutation test, Chinese hamster ovary cell chromosome aberration test, Chinese hamster gene mutation test and rat micronucleus test results were negative.
Reproductive toxicity:
Female rats were injected subcutaneously with 0.01, 0.03 or 0.1 mg/kg/day (AUC for 0.07, 0.2 and 1.2 times of 4 mg intravenously) from the 15th day before mating to the end of pregnancy. The high dose group showed ovulation inhibition and The conception rate is reduced. In the medium-dose and high-dose groups, the loss of embryos before implantation increased, the number of implanted embryos and the number of live fetuses decreased, and the survival rate of newborn rats decreased. Dysentery and perinatal mortality increased in all dose groups of mothers. The cause of death in the mother may be related to the drug's inhibition of bone calcium mobilization, leading to perinatal hypocalcemia, which may be a common role for bisphosphonates.
Female rats were injected subcutaneously with 0.1, 0.2 or 0.4 mg/kg/day (AUC for 1.2, 2.4 or 4.8 times when 4 mg was administered intravenously), and animals in medium and high doses were preimplanted or post-implanted. Increased loss, reduced number of live births, fetal bones, internal organs and appearance deformities. The skeletal malformations of the litters of high-dose animals showed no ossification and ossification, bone thickening, bending or shortening. In the high-dose group, there were also toxic reactions such as lens shrinkage, cerebellar hypoplasia, hepatic lobular shrinkage or loss, lung leaf deformation, vasodilation, cleft palate, and edema. Skeletal malformations also occur in low-dose animals. In this trial, the maternal animals in the high-dose group showed decreased body weight and food intake, suggesting that the trial has reached the highest drug exposure level.
The pregnant rabbits were given subcutaneously 0.01, 0.03, 0.1 mg/kg/day (the AUC was less than or equal to 0.5 times that of 4 mg intravenously), and no toxicity of the product to the litter was observed. Maternal death and abortion occurred in the animals of each drug group (converted to the relative body surface area and the dose was greater than or equal to 0.05 times the dose of 4 mg of human intravenous drug). This phenomenon may be related to hypocalcemia caused by drugs.
Carcinogenicity:
Conventional life-long carcinogenicity studies were conducted using mice and rats. The mice were orally administered with 0.1, 0.5, 2.0 mg/kg/day (according to the relative body surface area, the dose was greater than or equal to 0.002 times the amount of human intravenous drug 4 mg), and all the animals in the group were Harderian adenomas. The incidence has increased. The rats were orally administered 0.1, 0.5, 2.0 mg/kg/day (converted to the relative body surface area, the dose was less than or equal to 0.2 times the amount of 4 mg of human intravenous drug), and no increase in tumor incidence was observed.
【Pharmacokinetics】
Distribution
64 patients with cancer and bone metastases were given a single dose or multiple doses (4 times for 28 days) 2, 4, 8 or 16 mg, and the infusion time was 5 or 15 minutes. The concentration of zoledronic acid in the plasma decreased after the instillation. In the three-phase elimination process, the drop concentration is rapidly decreased from the peak concentration value, and the blood drug concentration is less than 1% of Cmax after 24 hours. The half-life of the first two phases was 0.25 hours for t1/2α and 1.87 hours for t1/2β. The final phase of zoledronic acid was cleared for a longer period of time, and remained low in plasma for 2 to 28 days after instillation. The final elimination half-life t1/2γ was 146 hours, and the area under the drug concentration-time curve (AUC0-24h) in plasma was proportional to the dose administered in the range of 2-16 mg. The accumulation rate of zoledronic acid in the three phases was low, and the ratios of the average AUC0-24h values of the two phases 3 to the first phase were 1.13±0.30 and 1.16±0.36, respectively. In vivo and in vitro tests showed that the affinity of zoledronic acid to human blood cells was low, and the binding rate to human plasma protein was about 22%, and the binding rate was independent of concentration.
2. Metabolism
In vitro tests showed that zoledronic acid had no inhibitory effect on human P450 enzyme, and zoledronic acid was not biotransformed in vivo, and was mainly excreted in the original form by the kidney.
Excretion
The average recovery rate of urine in 64 patients within 24 hours after administration of zoledronic acid was 39±16%. Only the trace amount of the drug was found in the urine on the second day after administration, and the urine was administered within 0-24 hours. The cumulative excretion percentage rate has nothing to do with the concentration of the drug. The drug recovery in the urine does not reach equilibrium within 0~24 hours. It is speculated that the drug first binds to the bone and then slowly releases into the systemic circulation, so that the observed long-term plasma is very long. The phenomenon of low concentration of drugs. The renal elimination rate of zoledronic acid was 3.7 ± 2.0 L/h within 0 to 24 hours after administration, and the clearance of zoledronic acid was dose-dependent and dependent on creatinine clearance. In one study, the infusion time for 4 mg of zoledronic acid in patients with cancer and bone metastases was extended from 5 minutes (n=5) to 15 minutes (n=7), resulting in the concentration of zoledronic acid at the end of the drip. A year-on-year decrease of 34% ([mean ± SD] 403 ± 118 ng / ml vs 264 ± 86 ng / ml), AUC total value increased by 10% (378 ± 116 ng × h / ml vs 420 ± 218 ng × h / ml) The difference in AUC values was not statistically significant.
【Storage】sealed and stored.
【Package】 vial, 1 bottle / box.
【Validity period】 18 months.
【Executive Standards】 National Food and Drug Administration National Drug Standard YBH21602004-2014Z
【Approval No.] National Drug Standard H20041979
【manufacturer】
Company Name: Shandong New Times Pharmaceutical Co., Ltd.
Production address: No. 1 North Outer Ring, Feixian County, Shandong Province
Postal code: 273400
Phone number: 0539-8336336 (Sales) 5030608 (Quality Management Department)
Fax number: 0539-5030900
Website: www.LUNAN.com.cn
24-hour customer service hotline: 400-0539-310
